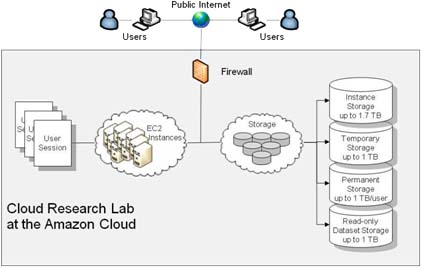
Cloud Research Lab
Using the Amazon Cloud, we created a centralized research facility for scientists to perform statistical epidemiological research.
Project Highlights:
- Over 100 scientists from around the world simultaneously conduct epidemiological research using our Research Lab.
- Data of over 100M patients are available within the Research Lab.
- The Lab allows the automation of treatment-outcome association analysis using 20 statistical methods
- 15 scientific papers have been published based on the conducted research
- The Lab allows the massively parallel launch of hundreds of experiments
- It is currently the largest SAS/R infrastructure on the cloud
- It provides HIPAA-compliant data security to safeguard sensitive Patient Health Information (PHI)
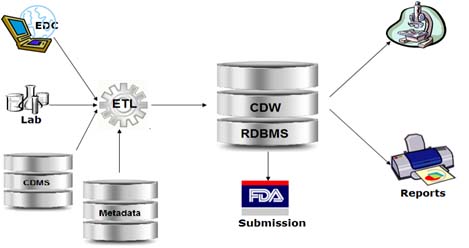
Clinical Data Warehouse
A real time data warehouse that consolidates information from a variety of clinical sources to present a unified view of clinical studies.
Project Highlights:
- Consolidated and formatted data such as patient demographics, ICD-9 encounters, lab results, pharmacy information, genomic data are integrated into a centralized data warehouse
- The CDISC standards-based warehouse is structured in ODM format.
- It allows to perform data aggregation and conversion to generate SDTM format of all collected information
- It supports the automation of manual processes of data conversion and the load to creation of submission material to FDA
- It provides multiple predefined visualizations
- It supports decision making through reporting and browsing: Patient Profiling, Summary Listings, cohort visualizations
- It allows to create tables and listings for reporting to FDA
- It has SAS Integration
- It is Part 11 compliant and fully documented for easy validation at the client site
Medical Observation Visualization
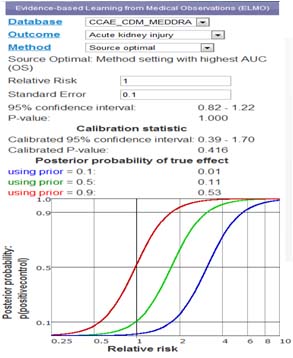
Data Visualization application provides real-time access to statistical results
Project Highlights:
- Real-time interaction and visualization of epidemiological experiments
- Ability to support large number of simultaneous users
- Support for computers via browser, iPhone, Android phones
- Data aggregated from thousands of experiments and millions data points
- Support for variety of DBMS’s
Gene Central
Centralized repository of gene information resource portal to facilitate research
Project Highlights:
- The solution provides mechanism to collect gene sequences and related information from private and public sources
- It creates comprehensive web services architecture access central gene database
- It automates and loads and distributes semi-manually gene sequences from corporate and open source resources
- It builds mechanisms for information extraction and search allowing third party developers to build interface software to other applications, e.g. Sequence Exploration, Data Mining, etc.
- The system performs gene annotation to mark up descriptive notations such as names, functions and biological features
- It is used at to five large pharmaceutical companies
Healthcare Projects Examples
Centralized repository of gene information resource portal to facilitate search and research
Project: Medical Bulletin Workflow
- We created a corporate workflow system to submit bulletin recommendations to doctors generated by prospective analytics (not in the chart)
- This provides a web-based mechanism to report PHI over Intranet and non-PHI information using extranet process
Project: Clinical Content Editor
- We developed applications that replaced a large set of paper-based medical SOPs for doctors and nurses with an electronic version
- The application distributed to over 80% of the hospitals in the US
Project: Health data collection and aggregation
- We created an automated solution to collect data from 40 large health organization. Organized data into a consistent architecture
- This provides a web based system management and reporting
Other developed Software Applicatuons
ComplianceFDA
- FDA Warning Letters, Regulations, and USCs cross-reference search engine
ClinVest
- Safety Letter Distribution
- Investigator Data Base
- Automatic distribution tracking
XPT Viewer/Utilities
- SAS export datasets conversion libraries API and utilities
TransReplicator
- DBMS replication.
- Masking proprietary information (e.g. HIPAA).
